Let’s Talk about Recognizing Macrosiphum euphorbiae (Thomas), the Potato Aphid
Andrew Jensen, Olathe, Colorado
January 2025
Introduction
In recent years this aphid is the one that I get the most questions about from researchers and extension agents doing field research or grower service projects. They want to be able to recognize “potato aphid” in trap samples and on host plants, and they hope to understand something about how it interacts with cultivated crops and its many acceptable host plants in agricultural environments. This is, however, one of the most difficult species to recognize with confidence in western North America, even for aphidologists. Persons without aphid taxonomy experience stand little chance of learning to recognize M. euphorbiae without outside help. With this essay I hope to compile some background information on the species, my thoughts about it, and some practical methods and advice for identifying specimens of probable M. euphorbiae.
A Native, Polyphagous Species
Macrosiphum euphorbiae was named by Cyrus Thomas in 1878. Below is what he wrote about it:
“8. Siphonophora euphorbiae, new sp.
Notes in reference to color lost. If I remember rightly, it was green or greenish, similar in color to the following species, but distinct.
Antennae longer than the body, very slender; the third and seventh joints very long; a few regularly placed hairs on them. Honey-tubes long and slender, reaching beyond the tip of the abdomen, nearly one third as long as the body, cylindrical. Tail very distinct, ensiform and slender, about half the length of the honey-tubes. Subcostal vein of the front wings diverging as it leaves the base, so as to leave the widest space between it and the costal vein opposite the insertion of the first discoidal vein, then approaching and joining it at the stigma.
Found at Sioux City, Iowa, September 1st, on Euphorbia maculata.“
I always like reading these old papers, seeing the alternative names they used for body parts, and noting that their descriptions are completely useless in modern aphid taxonomy (Heck, descriptions like this were useless the day they were written. Thomas and others can hardly be faulted, though, because they had no idea how much aphid diversity would eventually be uncovered, plus they were working on numerous insect groups as they attempted to document diversity in newly conquered lands.) Thomas used “honey-tubes” for our siphunculus or cornicle, and “tail” for cauda. He also interpreted the antennae as 7-segmented, considering the base of VI (BASE) and the processus terminalis (PT) as separate segments.
This species is now distributed throughout most of the world. I am quite confident, however, that it is native to North America, and is most evident and influential in the northwestern third of the U.S. and adjacent Canada. The reason I assert this is that M. euphorbiae can be found in practically every habitat type in the western states: ocean coasts, undisturbed forests, cities, crops, deserts, to mountain slopes above tree line. Its remarkable reach and flexibility suggest to me that it has had a long time to adapt to and spread throughout the diversity of habitats and geography of western North America.
Macrosiphum euphorbiae is one of the most polyphagous aphid species alive today. It has been recorded reproducing on hundreds of plant species, with no end in sight to discovery of new acceptable hosts. In my collection are 462 slides of this species, recorded from 132 different hosts from the ocean coast of Washington State to about 4,000 meters elevation in the Rocky Mountains of Colorado. Although it mostly inhabits dicots, it can feed on monocots including crops. One especially bizarre find, which I left in the Oregon State University collection, was on grain corn (maize) on a research station in eastern Oregon. Here the aphids were a creepy white-translucent color and lived on the corn leaves deep in the field.
Because of its polyphagy, it is not possible to use our typical means of aphid identification, which is referring to the list of species known to feed on a given plant genus and running through a key to those species. New hosts of M. euphorbiae are discovered all the time, so we cannot eliminate it as a possibility on most plant hosts. Therefore, we must be able to recognize this species based solely on morphology.
The life cycle of M. euphorbiae is generally heteroecious-holocyclic with various Rosa species as primary host. As I have seen, and many authors have noted, oviparae, males, and eggs can often be found on various herbaceous secondary hosts. This leads one to think the species may simply be polyphagous and not heteroecious. I have never collected a fundatrix from any herbaceous plant, however, despite my nearly 30 years of experience collecting aphids. So, my sense is that the aphid can produce oviparae and eggs on herbs but the eggs and fundatrices rarely if ever succeed except on Rosa. This aphid can also be anholocyclic on numerous herbaceous plants. Just last year we found it living on volunteer lettuce plants in our western Colorado yard in late April, long before its heteroecious life cycle could have allowed this. (Incidentally, finds like this point to the fact that aphids can be anholocyclic not just in places with mild, green winters. All that is required is a host plant that stays vegetative with tender soft growth all winter. Plants like lettuce are ideal because they can survive extreme cold and stay green all winter, growing whenever weather permits. It is fallacious to think that aphids are killed by freezing temperatures. What kills them is lack of acceptable host plant material, which can be caused by cold weather.)
Variability in Color, Behavior, and Morphology
Like other polyphagous aphid species with broad habitat adaptation (e.g., Aphis gossypii Glover, Myzus persicae Sulzer), M. euphorbiae is frustratingly variable in color, size, morphology, and appearance/behavior on the plant. A bunch of the color variation in life can be seen here: https://aphidtrek.org/?page_id=21#m-euphorbiae. Size variation I have seen easily encompasses 3.3 mm body length on the large end to 1.8 mm on the small end. Degree of pigmentation in cleared and mounted specimens can also vary quite a bit, especially in terms of pigmentation of the cauda and siphunculi, which are often darker in specimens that were living in cool habitats or times of year. For example, when I was a postdoc in Maryland in the late 1990s I collected a Macrosiphum through much of the winter on cultivated Verbena. As the winter approached the specimens got darker green and their siphunculi and caudas got darker and darker. While it was tempting to identify these as a different species, such as the enigmatic and quite probably imaginary Macrosiphum verbenae (Thomas), I ended up concluding that they were just M. euphorbiae changing appearance with the season.
Interestingly, I also see variability in behavior in the field. Some is relatively subtle, like aphids living on growing tips (most common) versus lower yellowing leaves (uncommon, but an example is specimens I sometimes find on old leaves of Heracleum lanatum). Other behavior variability is more dramatic. I think the most common way to witness M. euphorbiae on its hosts is relatively small groups of one or a few adult females with a scattering of offspring, all on growing tips, expanding leaves, etc. They usually move little unless their plant is hit, stepped on, or similar. In contrast, when aphids identified as M. euphorbiae live on Osmorhiza (sweet cicely) they spread out along the freshest flower stalk stems, and they often wag their bodies vigorously as they sense me approach. This body wagging is a common defensive behavior among many species of Macrosiphini with long appendages. When I first witnessed this, I was prompted to seriously consider that specimens on Osmorhiza may represent a distinct species. But, after years of collecting and considering I don’t think the evidence is there to split off a species.
It is also important to comment on variable morphology in taxonomically important features. I will get into this subject in the next section about how I recognize M. euphorbiae.
So how do I recognize it then??
It might seem a contradiction that I am writing an entire essay about how challenging this species is, while also telling you that I’ve identified 462 of my slides as M. euphorbiae. If it is so hard to determine confidently, and I brashly say that an unexperienced person will find it nigh impossible, what features do I use to put this name on so many samples? That’s a simple question with a complicated answer. Before going on to answer this question, let me clarify that clearing, slide mounting, and a compound microscope equipped with phase contrast optics and at least 160X magnification (i.e., a 16x or greater objective with 10x eye pieces) are required to learn M. euphorbiae identification as I describe it below.
First, we must confirm that a specimen is a species of Macrosiphum. Among aphids in western North America, this is reasonably easy. We can see from my Macrosiphum page on this website that most species are elongate, large (for aphids), and have long appendages, including siphunculi and cauda. These features are common to members of many genera in our region, including Acyrthosiphon, Amphorophora, Bipersona, Delphiniobium, Ericaphis, Illinoia, Macrosiphoniella, Macrosiphum, Metopolophium, Microlophium, Obtusicauda, Sitobion, Uroleucon, Wahlgreniella, and the list could go on. This might sound like a lot of genera, and it is, but if we had to consider all genera worldwide, it would be much more challenging. That’s why I say it is relatively easy to recognize Macrosiphum in the context of western North America.
The single most important feature to be able to see and understand in your specimens is apical reticulation of the siphunculi. Macrosiphum euphorbiae and most other Macrosiphum species have about 4 to 8-ish rows of polygonal reticulation at the apex of the siphunculus (e.g., Figure 1). This feature eliminates many of the possibilities listed above and some others not listed. The next most important consideration is how big are the individual cells if reticulation? Smaller cells indicate genera other than Macrosiphum such as Macrosiphoniella, Uroleucon, and Sitobion. See Figure 2 for a typical example of Uroleucon reticulation; compare this to Figure 1.
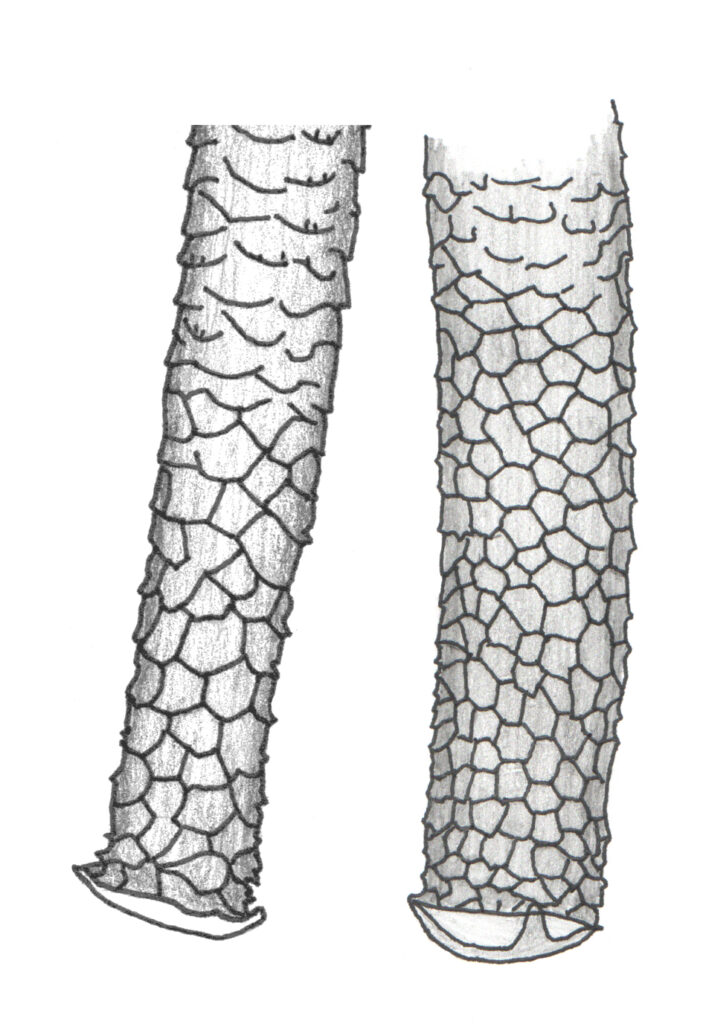
If you see this larger form of reticulation, you probably have a Macrosiphum (there are, of course, exceptions to this rule just as there are exceptions to almost all rules in aphid taxonomy and classification, but exceptions can mostly be ignored when learning to recognize just this one species). Other features should probably be checked too. Are the siphunculi swollen (a.k.a. clavate; Figure 3) on the apical half? If yes, then you don’t have M. euphorbiae.
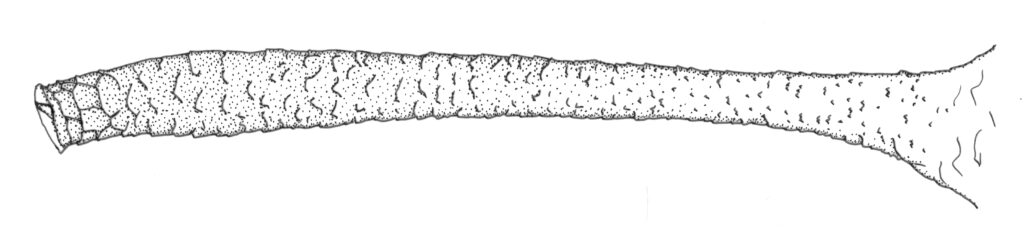
Is the cauda long, straight, nearly parallel sided, with about 10-14 hairs (alternatives include more hairs, a twisted curved cauda, or a shortish triangular cauda)? Is the apical rostral segment (R IV+V) more or less parallel-sided, gradually tapering, with somewhere around 12-18 total hairs? If yes to both, we are now in the ballpark of M. euphorbiae.
Hooray, we are getting close! The easy features have been confirmed! We probably have a Macrosiphum and we probably have one that is either M. euphorbiae or that is sort of similar to it. Now what?
First of all, I am assuming that we have before us a nicely prepared slide with cleared specimens including at least one each apterous and alate viviparous female. If we lack both morphs, our identification guess will simply be less confident. I’ll cover what we can do with isolated apterae and alatae later.
Step 1 is to check the distribution and number of secondary rhinaria on the antennal segment III (AS III). In the apterae there should be a “handful”, perhaps 2-8, more or less clustered near the base. The number and distribution of rhinaria can be affected by time of year, overall size of the specimen, and possibly other issues. If rhinaria are much more than 8 and/or they are distributed beyond the basal half of AS III, then it is worthwhile checking for vestigial lateral ocelli or deformation of the thorax that might indicate that the specimen is an intermediate aptera/alata, a common phenomenon. Next, we check the rhinaria on AS III in the alate specimen. If sensoria are scattered evenly (about 15-20 total) over approximately the basal 4/5 of the segment, then we might have M. euphorbiae. If rhinaria are distributed to the tip of AS III then we definitely do not have M. euphorbiae. Rhinaria in alatae of M. euphorbiae can occasionally be fewer and restricted to as little as the basal half of AS III.
Assuming antennal rhinaria conform to these criteria, I would next check relative lengths of the R IV+V, BASE, and HT II (second segment of hind tarsus). All three of these are more or less equal in M. euphorbiae, “more or less” being important. The BASE is usually going to be a bit longer than the R IV+V and HT II, but they will all be close to the same. What constitutes ‘close” requires experience and judgement, but 10% variation is a good guideline.
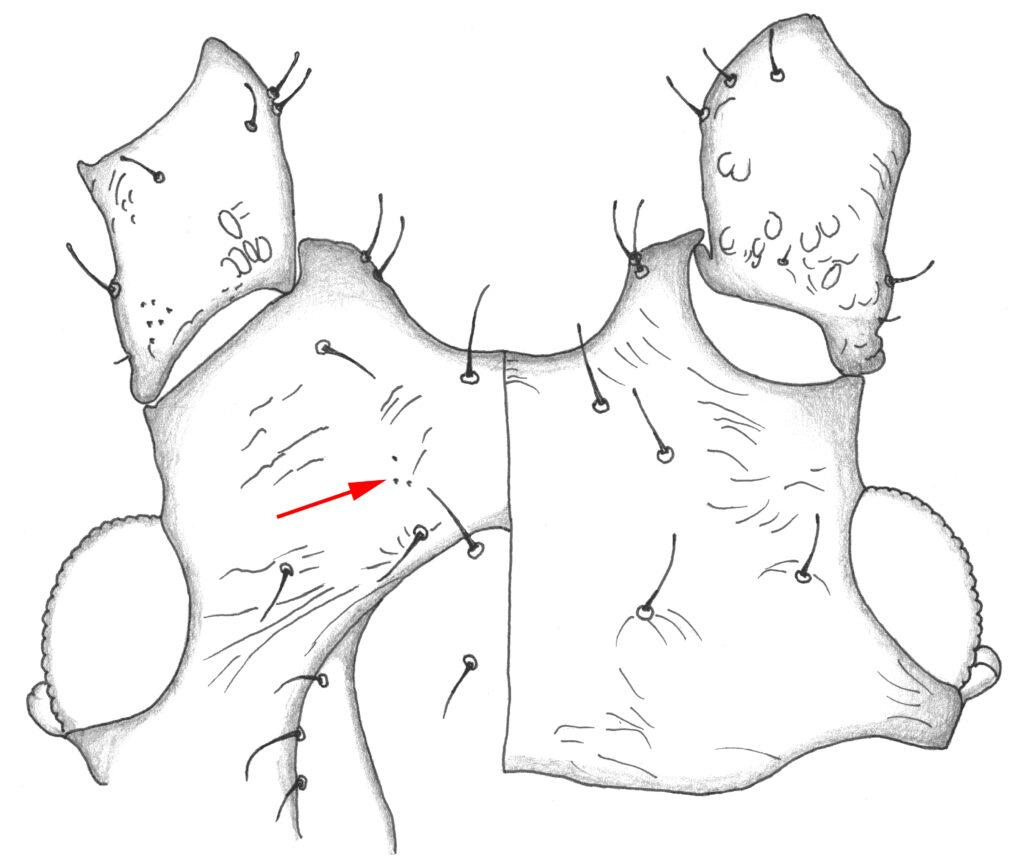
Next, I would check for small lateral tubercles at the base of the siphunculi. Apterae and alatae almost always have these in M. euphorbiae. Then we need to look at the ventral surface of the head of the apterae, in the space between the clypeus and antennae. On each side in this space M. euphorbiae almost always has a very small patch of what I call spinules – extremely small sharp-looking protrusions (Figure 4). Most Macrosiphum species have some ventral spinules on the head, but M. euphorbiae is one of very few that has this tiny patch of spinules (2-5 in most specimens) in this location.
After confirming these key features, it is also necessary to look at the cauda. How many hairs does it have and how are they distributed? In M. euphorbiae the cauda usually has 4 or 5 lateral hairs on each side plus one to a few hairs dorsally in the apical ~½. Other species may have only 3 lateral hairs on each side, many more than 5, or only one, or more than 3 or 4, dorsal hairs.
As I look at each specimen, I am scanning for anything else unusual. Are the hairs on body or appendages unusually short or long (M. euphorbiae hairs are medium-sized in the context of Macrosiphum taxonomy)? Is the cauda oddly narrow or broad (M. euphorbiae is medium)? Are there spinal tubercles on the head, prothorax, and/or abdominal tergites (M. euphorbiae almost always lacks these)? How many hairs are on the first tarsal segments (M. euphorbiae has 3)? I confirm that the abdominal dorsum is membranous; many Macrosiphum species have a pale but thick sclerotized tergum. Macrosiphum euphorbiae and most similar relatives have a thin membranous dorsum; this can be determined by looking for folds or wrinkles, whereas a scerotized tergum will not be folded or wrinkled by the mounting process except in extreme cases of man-handling or over-clearing.
Some of you might wonder why I list no other characters that are ratios of one body part’s length to another. The reason is that in my experience such ratios are rarely useful across the full range of variation of a species. To destroy the utility of such characters, all one usually must do is collect a species in numerous places and times of year. I should write an essay on this subject someday.
Morphologically Similar Species
In western North America there are several species of Macrosiphum that have been demonstrated to be distinct species biologically but that are very similar to, or effectively indistinguishable from, M. euphorbiae morphologically. I want to discuss some of these, just to illustrate the point.
Macrosiphum agrimoniellum (Cockerell) is a name I’ve used on a handful of samples from New Mexico and Colorado. In life it looks like M. euphorbiae with somewhat darker than normal legs and siphunculi, and is known from Agrimonia. Although Blackman and Eastop report this species to have 15-20 rhinaria on ANT III in apterae, I’m not sure that is always the case. If I am right about this, then M. euphorbiae and M. agrimoniellum can be extremely similar. Another complicating factor is that I have collected what appears to be this species from Potentilla in both New Mexico and Colorado. In my few specimens it appears that M. agrimoniellum differs from M. euphorbiae by lacking lateral tubercles at the base of the siphunculi, trending toward fewer hairs on the cauda, and the R IV+V seems a bit longer relative to the HT II and it has oddly long and thick accessory hairs.
Macrosiphum corydalis (Oestlund) is a species that I have waffled about over the years – is it just small M. euphorbiae living on small members of Fumariaceae in rocky outcrops or is it a good species? Some years ago I decided it seemed like a good species. It differs from M. euphorbiae mainly in features that vary with size in this group of aphids. For example, the AS III is short and in alatae only has about 10 rhinaria that sometimes are distributed almost to the tip. The body is overall small compared to typical M. euphorbiae. The dorsal setae on the abdomen are unusually short in most specimens (a feature I have never quantified but is evident upon reviewing several specimens). The lateral tubercles at the base of siphunculi are usually present, and the cluster of spinules on the ventral surface of the head is much like in M. euphorbiae.
Macrosiphum creelii (Davis) feeds on legumes across most of North America and looks very much like M. euphorbiae. Once cleared and mounted, it is fairly easy to separate from M. euphorbiae by the longer BASE, about twice as long as the R IV+V, the tarsi tending to be longer, close to the BASE in length, and the antennae, cauda, and head just giving the impression of being bigger and thicker. The small patch of spinules on the ventral surface of the head and small lateral tubercles at the base of the siphunculi are essentially just like M. euphorbiae.
Macrosiphum mertensiae Gillette and Palmer is doubtless one of the most challenging species to identify once cleared and mounted. In life, it is convincingly different from M. euphorbiae, living on the lower leaves of large species of Mertensia, usually being white in life, and just looking different in the field. When mounted, however, there is frustratingly little to separate it from M. euphorbiae. The key characters used by Blackman and Eastop definitely are inadequate. They say:
4 ANT III with 7-11 rhinaria extending over more than 0.5 of length. R IV with 6 accessory hairs…..Macrosiphum mertensiae
–ANT III with 1-10 (usually 2-6) rhinaria extending over less than 0.5 of length. R IV with 7-10 accessory hairs…..Macrosiphum euphorbiae
Both of the features used for M. mertensiae do not apply to any of my specimens. Of course, it is possible that I am not looking at M. mertensiae, but I don’t think I’m wrong. About the only features I see that sort of consistently separate these two species are that in M. mertensiae alatae the rhinaria on ANT III are distributed farther toward the tip of the segment, ANT III and siphunculi look more densely imbricated than M. euphorbiae, and the antennae in apterae are more consistently pale except for narrow bands of pigments at the joints. That said, I think isolated specimens without host plant information and live color notes would be impossible to identify consistently.
Macrosiphum valerianae (Clarke) is a very interesting case. Because of its dark red-brown color and its dark appendages, it is unlikely to be confused with M. euphorbiae. However, as I wrote in my 2012 paper, “The key to aphids on Geum in Blackman and Eastop (2006) shows simple ways to separate this species from similar Macrosiphum in North America. The key will work well with most specimens of M. valerianae, but like many aphid species, there will be specimens on the extreme ends of the range of variation that will not fit. The most troubling species pair will likely be M. valerianae and Macrosiphum euphorbiae (Thomas). Both species are variable, and dark specimens of M. euphorbiae exist that might key to M. valerianae, likewise pale specimens of M. valerianae that might key to M. euphorbiae. This confusion would be especially likely if the color in life of a specimen was not known. Other characters that are usually consistent include, lack of lateral tubercles near the base of the siphunculi in M. valerianae, whereas M. euphorbiae normally has lateral tubercles (except in small specimens); apterae of M. valerianae usually have distinct (but sometimes very pale) presiphuncular sclerites, even in relatively pale specimens, while M. euphorbiae lacks these.”
In addition to these examples, there are a few forms that I think are undescribed species on unusual host plants (e.g., Agastache, Monardella) that seem like good species based on field observations but that are frustratingly variable and similar to M. euphorbiae once cleared and mounted.
Identification of isolated apterae, alatae in traps, and samples from unusual host plants
Aphid taxonomists are going to be challenged to identify M. euphorbiae specimens that come with no host plant information, that come from host plants not previously recorded, and from traps. Below are some of my thoughts on these scenarios.
Apterae without host plant information (or from previously unrecorded hosts) and without accompanying alatae are especially difficult to recognize as M. euphorbiae. If we don’t know the host plant, then we don’t know whether there are other Macrosiphum species known from it that we must compare to M. euphorbiae. As mentioned above, for example, I think confident identification of M. euphorbiae from Mertensia species would be nearly impossible without host plant information, and is very challenging with it. Apterae without alatae are especially difficult because we are left without a key feature of M. euphorbiae: the distribution of rhinaria on AS III. So, can we be confident of identifications of isolated apterae of M. euphorbiae? I don’t think so. All we can confidently say is that a specimen is or is not very similar to, and may in fact be, M. euphorbiae.
A similar conclusion must be made when looking at alate specimens from traps. Assuming we have adequate lighting and magnification to see the important characters of siphunculi and antennae (no small caveat – my dissecting scope is inadequate without use of imagination, intuition, or experience), we can again declare specimens to be either M. euphorbiae or similar to it. There is no way to be sure what species we have. I think that all research and extension projects reporting on flights of M. euphorbiae should be honest and state that their identifications based on morphology are only educated guesses and that it is possible that some determinations were incorrect (of course such caveats should be stated for many species of aphids we are tempted to identify from traps).
As a natural historian and avid aphid collector, I am often confronted with samples of Macrosiphum from plants that present aphid identification challenges for some reason. For example, the plant may have no previous records of any aphids, or no previous records of any Macrosiphum, or no previous samples of M. euphorbiae. The first clues that I use to point me toward Macrosiphum species identification are to do with appearance in life and position/behavior on the plant. Sometimes I find Macrosiphum species that look like M. euphorbiae in life except for extra pigmentation on the legs or antennae, or they may be white or bright yellow, or they may be living on old senescing leaves, or they may be living in a habitat not normally favored by M. euphorbiae, such as desert scrubland. These clues suggest that I should carefully examine the specimens during and after mounting for morphological clues about species identity. One might also see strange behavior, like the body-wagging I mentioned above in specimens seen on Osmorhiza. Anything different from what I’m used to seeing M. euphorbiae do and look like are clues that I may have something different. “May” here carries a lot of water. Just because something is different from typical M. euphorbiae in these regards does not mean that it is not M. euphorbiae. With concerted collecting effort we will continue to discover new host plants and situations acceptable to M. euphorbiae, and new similar but biologically distinct species, for many years to come.
References Cited
Blackman, R.L. and Eastop, V.F. (2006) Aphids on the World’s Herbaceous Plants and Shrubs. John Wiley & Sons, Ltd., West Sussex. 1439 pp.
Hille Ris Lambers. 1939. Contributions to a monograph of the Aphididae of Europe II. The genera Dactynotus Rafinesque, 1818; Staticobium Mordvilko, 1914; Macrosiphum Passerini, 1860; Masonaphis nov. gen.; Pharalis Leach, 1826. Temminckia 4:1-134
Jensen, A.S. 2012. Macrosiphum (Hemiptera: Aphididae) Update: One New Species, One Synonymy, and Life Cycle Notes. Proceedings of the Entomological Society of Washington 114: 205-216.
Thomas, Cyrus. 1878[1877]. A list of the species of the tribe Aphidini, family Aphidae, found in the United States, which have heretofore been named, with descriptions of some new species. Bulletin of the Illinois State Laboratory of Natural History 1(2):3-16.
